UNDATED (AP) — Johnson & Johnson’s COVID-19 vaccine will remain in limbo a while longer.
Distribution of the virus remains on hold after government health advisers said they need more evidence to decide if a handful of unusual blood clots were linked to the shot — and if so, how big the risk really is.
The reports are exceedingly rare — six cases out of more than 7 million U.S. inoculations with the one-dose vaccine.
But the government has recommended a pause in J&J shots this week. The move came soon after European regulators declared that such clots are a rare but possible risk with the AstraZeneca vaccine. The AstraZeneca shot is made in a similar way as the J&J vaccine — and is not yet approved for use in the U.S.
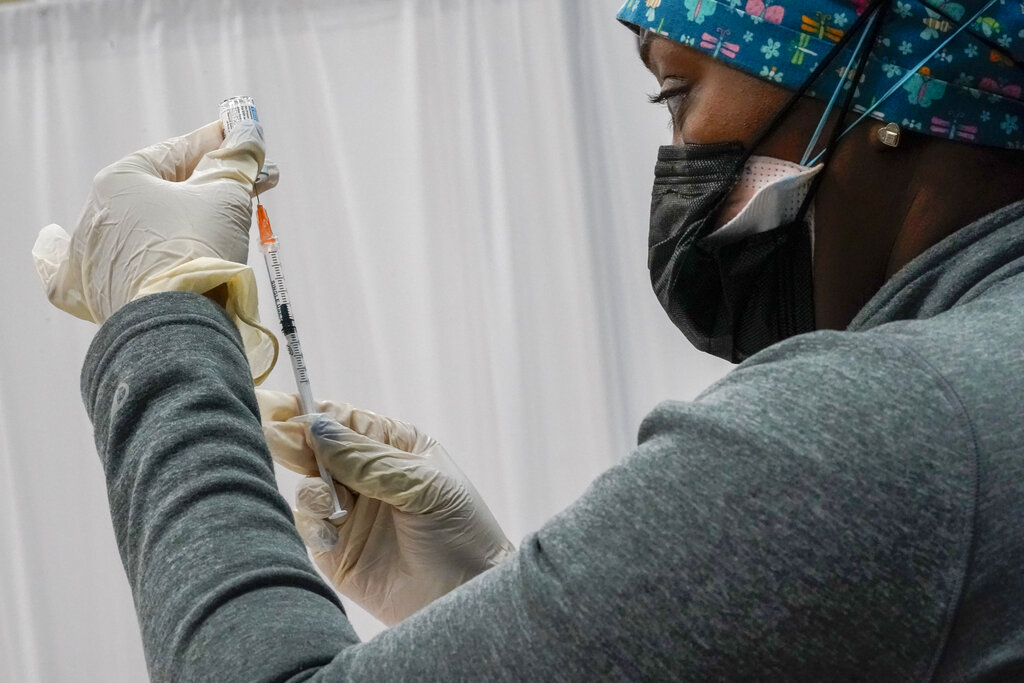
Photo: A Northwell Health registered nurse fills a syringe with the Johnson & Johnson COVID-19 vaccine at a pop up vaccination site inside the Albanian Islamic Cultural Center, Thursday, April 8, 2021, in the Staten Island borough of New York. (AP Photo/Mary Altaffer)